Ben-Shachar 2009 J Neural Transm (Vienna)
| Ben-Shachar D (2009) The interplay between mitochondrial complex I, dopamine and Sp1 in schizophrenia. J Neural Transm (Vienna) 116:1383-96. https://doi.org/10.1007/s00702-009-0319-5 |
Ben-Shachar D (2009) J Neural Transm (Vienna)
Abstract: Schizophrenia is currently believed to result from variations in multiple genes, each contributing a subtle effect, which combines with each other and with environmental stimuli to impact both early and late brain development. At present, schizophrenia clinical heterogeneity as well as the difficulties in relating cognitive, emotional and behavioral functions to brain substrates hinders the identification of a disease-specific anatomical, physiological, molecular or genetic abnormality. Mitochondria play a pivotal role in many essential processes, such as energy production, intracellular calcium buffering, transmission of neurotransmitters, apoptosis and ROS production, all either leading to cell death or playing a role in synaptic plasticity. These processes have been well established as underlying altered neuronal activity and thereby abnormal neuronal circuitry and plasticity, ultimately affecting behavioral outcomes. The present article reviews evidence supporting a dysfunction of mitochondria in schizophrenia, including mitochondrial hypoplasia, impairments in the oxidative phosphorylation system (OXPHOS) as well as altered mitochondrial-related gene expression. Abnormalities in mitochondrial complex I, which plays a major role in controlling OXPHOS activity, are discussed. Among them are schizophrenia specific as well as disease-state-specific alterations in complex I activity in the peripheral tissue, which can be modulated by DA. In addition, CNS and peripheral abnormalities in the expression of three of complex I subunits, associated with parallel alterations in their transcription factor, specificity protein 1 (Sp1) are reviewed. Finally, this review discusses the question of disease specificity of mitochondrial pathologies and suggests that mitochondria dysfunction could cause or arise from anomalities in processes involved in brain connectivity.
• Bioblast editor: Gnaiger E
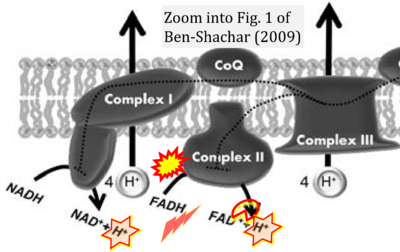
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.