| Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. https://doi.org/10.1016/S0034-5687(01)00307-3 |
» Respir Physiol 128:277-97. PMID: 11718759, ![]()
Gnaiger Erich (2001) Respir Physiol
Abstract: Oxygen limitation is generally considered as impairment of mitochondrial respiration under hypoxia and ischemia. Low intracellular oxygen levels under normoxia, however, imply mild oxygen limitation, provide protection from oxidative stress, and result from economical strategies for oxygen transport through the respiratory cascade to cytochrome c oxidase. Both perspectives relate to the critical oxygen pressure which inhibits mitochondrial respiration. Based on methodological considerations of oxygen kinetics and a presentation of high-resolution respirometry, mitochondrial oxygen affinities (1/p50) are reviewed with particular emphasis on the turnover effect under control of ADP, which increases the p2 in active states. ~P/O2 flux ratios are high even under severe oxygen limitation, as demonstrated by calorespirometry. Oxygen limitation reduces the uncoupled respiration observed under control by ADP, as shown by relationships derived between ~P/O2 flux ratios, respiratory control ratios, and ADP kinetics. Bioenergetics at low oxygen versus oxidative stress must be considered in the context of limitation of maximum aerobic activity, ischemia-reperfusion injury, mitochondrial signalling to apoptosis, and mitochondrial theories of ageing.
• Keywords: Energy: Oxidative phosphorylation, Adenosine diphosphate kinetics, Adenosine diphosphate/O2 ratio; Hypoxia: Mitochondrial O2 kinetics, Mammals: Rat, Membrane permeability, Mitochondria: Heart, Liver
• O2k-Network Lab: AT Innsbruck Oroboros
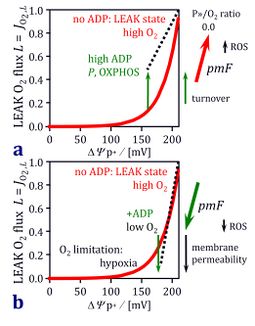
Figure 10 (2nd ed): Opposite effects of ADP limitation and oxygen limitation on mitochondrial membrane potential or the protonmotive force pmF and LEAK-compensating O2 flux L. In the presence of ADP, L is the noncoupled component of OXPHOS capacity. (a) ADP limitation of respiration at high oxygen levels in the transition from the active OXPHOS state to the resting LEAK state leads to an increase of ΔΨp+ and exponential acceleration of the proton leak (heavy line). Because uncoupled O2 flux increases while total O2 flux is reduced, the ATP yield declines to zero. Turnover-dependent proton leaks increase the uncoupled O2 flux in the OXPHOS state but decline towards the LEAK state. Mitochondrial production of reactive oxygen species (ROS) increases with pmF towards the LEAK state, and ROS-linked electron bypass contributes minimally to uncoupled O2 flux at high oxygen. On the right, the decline of P»/O2 flux ratios is shown in the transition from the OXPHOS to the LEAK state (from Figure 9b). (b) Oxygen limitation of respiration causes a reduction of ΔΨp+ in the transition from ADP limitation at high oxygen, to intracellular conditions of low oxygen and low ADP, to finally severe oxygen limitation under hypoxia and anoxia. Potentially synergistic with the well documented ΔΨp+ effect on uncoupled flux, are the hypothetical effects of decreasing membrane permeability and suppression of ROS production under hypoxia.
* Discussion: Oxygen dependence of ROS production - are permeabilized fibers a valid model?.
Selected quotes
- In the intracellular microenvironment, mitochondria are well separated from air-level oxygen pressure, and high rates of oxidative phosphorylation must be maintained near or at limiting oxygen levels in some tissues. On the other hand, mitochondria are routinely isolated and studied at unphysiologically high oxygen concentrations with limited additions of antioxidants, despite the fact that mitochondria in tissues are protected from oxidative stress by both low oxygen levels and complex defence systems against reactive oxygen species.
- Mitochondrial p50 values are 5 to 20 times less than the half-saturation point of myoglobin, which then suggests that mitochondrial respiration operates at the edge of oxygen limitation under normoxic intracellular oxygen pressures (Gnaiger et al 1998b).
Harmonization of selected symbols
Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1
- rate in State 4 → L
- rate in State 3 → P, provided that ADP concentration is kinetically saturating
- RCR → P/L
- YP/O2 → P»/O2
- P/O2 → νP»/O2
- JADP → JP»[D] = RP» (Eq. 13)
- JADP,max → PP» (Eq. 13)
- JO2 → JO2[D] = R (Eq. 14)
- JO2,max → P (Eq. 14)
- intercept a → L° (Figure 8a, 9)
- RCRADP/a → R/L°
- RCRADP/a,max → P/L°
Keywords: Oxia terms
- Bioblast links: Hypoxia, normoxia, hyperoxia - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
| Term | Abbreviation | Description |
|---|---|---|
| Aerobic | ox | The aerobic state of metabolism is defined by the presence of oxygen (air) and therefore the potential for oxidative reactions (ox) to proceed, particularly in oxidative phosphorylation (OXPHOS). Aerobic metabolism (with involvement of oxygen) is contrasted with anaerobic metabolism (without involvement of oxygen): Whereas anaerobic metabolism may proceed in the absence or presence of oxygen (anoxic or oxic conditions), aerobic metabolism is restricted to oxic conditions. Below the critical oxygen pressure, aerobic ATP production decreases. |
| Anaerobic | Anaerobic metabolism takes place without the use of molecular oxygen, in contrast to aerobic metabolism. The capacity for energy assimilation and growth under anoxic conditions is the ultimate criterion for facultative anaerobiosis. Anaerobic metabolism may proceed not only under anoxic conditions or states, but also under hyperoxic and normoxic conditions (aerobic glycolysis), and under hypoxic and microxic conditions below the limiting oxygen pressure. | |
| Anoxia | anox | Ideally the terms anoxia and anoxic (anox, without oxygen) should be restricted to conditions where molecular oxygen is strictly absent. Practically, effective anoxia is obtained when a further decrease of experimental oxygen levels does not elicit any physiological or biochemical response. The practical definition, therefore, depends on (i) the techiques applied for oxygen removal and minimizing oxygen diffusion into the experimental system, (ii) the sensitivity and limit of detection of analytical methods of measuring oxygen (O2 concentration in the nM range), and (iii) the types of diagnostic tests applied to evaluate effects of trace amounts of oxygen on physiological and biochemical processes. The difficulties involved in defining an absolute limit between anoxic and microxic conditions are best illustrated by a logarithmic scale of oxygen pressure or oxygen concentration. In the anoxic state (State 5), any aerobic type of metabolism cannot take place, whereas anaerobic metabolism may proceed under oxic or anoxic conditions. |
| Critical oxygen pressure | pc | The critical oxygen pressure, pc, is defined as the partial oxygen pressure, pO2, below which aerobic catabolism (respiration or oxygen consumption) declines significantly. If anaerobic catabolism is activated simultaneously to compensate for lower aerobic ATP generation, then the limiting oxygen pressure, pl, is equal to the pc. In many cases, however, the pl is substantially lower than the pc. |
| Hyperoxia | hyperox | Hyperoxia is defined as environmental oxygen pressure above the normoxic reference level. Cellular and intracellular hyperoxia is imposed on isolated cells and isolated mitochondria at air-level oxygen pressures which are higher compared to cellular and intracellular oxygen pressures under tissue conditions in vivo. Hyperoxic conditions may impose oxidative stress and may increase maximum aerobic performance. |
| Hypoxia | hypox | Hypoxia (hypox) is defined in respiratory physiology as the state when insufficient O2 is available for respiration, compared to environmental hypoxia defined as environmental oxygen pressures below the normoxic reference level. Three major categories of hypoxia are (1) environmental hypoxia, (2) physiological tissue hypoxia in hyperactivated states (e.g. at VO2max) with intracellular oxygen demand/supply balance at steady state in tissues at environmental normoxia, compared to tissue normoxia in physiologically balanced states, and (3) pathological tissue hypoxia including ischemia and stroke, anaemia, chronic heart disease, chronic obstructive pulmonary disease, severe COVID-19, and obstructive sleep apnea. Pathological hypoxia leads to tissue hypoxia and heterogenous intracellular anoxia. Clinical oxygen treatment ('environmental hyperoxia') may not or only partially overcome pathological tissue hypoxia. |
| Intracellular oxygen | pO2,i | Physiological, intracellular oxygen pressure is significantly lower than air saturation under normoxia, hence respiratory measurements carried out at air saturation are effectively hyperoxic for cultured cells and isolated mitochondria. |
| Limiting oxygen pressure | pl | The limiting oxygen pressure, pl, is defined as the partial oxygen pressure, pO2, below which anaerobic catabolism is activated to contribute to total ATP generation. The limiting oxygen pressure, pl, may be substantially lower than the critical oxygen pressure, pc, below which aerobic catabolism (respiration or oxygen consumption) declines significantly. |
| Microxia | microx | Microxia (deep hypoxia) is obtained when trace amounts of O2 exert a stimulatory effect on respiration above the level where metabolism is switched to a purely anaerobic mode. |
| Normoxia | normox | Normoxia is a reference state, frequently considered as air-level oxygen pressure at sea level (c. 20 kPa in water vapor saturated air) as environmental normoxia. Intracellular tissue normoxia is variable between organisms and tissues, and intracellular oxygen pressure is frequently well below air-level pO2 as a result of cellular (mainly mitochondrial) oxygen consumption and oxygen gradients along the respiratory cascade. Oxygen pressure drops from ambient normoxia of 20 kPa to alveolar normoxia of 13 kPa, while extracellular normoxia may be as low as 1 to 5 kPa in solid organs such as heart, brain, kidney and liver. Pericellular pO2 of cells growing in monolayer cell cultures may be hypoxic compared to tissue normoxia when grown in ambient normoxia (95 % air and 5 % CO2) and a high layer of culture medium causing oxygen diffusion limitation at high respiratory activity, but pericellular pO2 may be effectively hyperoxic in cells with low respiratory rate with a thin layer of culture medium (<2 mm). Intracellular oxygen levels in well-stirred suspended small cells (5 - 7 mm diameter; endothelial cells, fibroblasts) are close to ambient pO2 of the incubation medium, such that matching the experimental intracellular pO2 to the level of intracellular tissue normoxia requires lowering the ambient pO2 of the medium to avoid hyperoxia. |
- General
- Related keyword lists
Publications: Tissue normoxia
| Year | Reference | Organism | Tissue;cell | Preparations | Stress | Diseases | |
|---|---|---|---|---|---|---|---|
| Donnelly 2022 BEC | 2022 | Donnelly C, Schmitt S, Cecatto C, Cardoso LHD, Komlódi T, Place N, Kayser B, Gnaiger E (2022) The ABC of hypoxia – what is the norm. Bioenerg Commun 2022.12.v2. https://doi.org/10.26124/bec:2022-0012.v2 | Oxidative stress;RONS Hypoxia | ||||
| Donnelly 2022 MitoFit Hypoxia | 2022 | Donnelly C, Schmitt S, Cecatto C, Cardoso LHD, Komlodi T, Place N, Kayser B, Gnaiger E (2022) The ABC of hypoxia – what is the norm. https://doi.org/10.26124/mitofit:2022-0025.v2 — 2022-11-14 published in Bioenerg Commun 2022.12. | Oxidative stress;RONS Hypoxia | ||||
| DiProspero 2021 Toxicol In Vitro | 2021 | DiProspero TJ, Dalrymple E, Lockett MR (2021) Physiologically relevant oxygen tensions differentially regulate hepatotoxic responses in HepG2 cells. https://doi.org/10.1016/j.tiv.2021.105156 | Liver | Intact cells | Hypoxia | ||
| Stepanova 2020 Methods Cell Biol | 2020 | Stepanova A, Galkin A (2020) Measurement of mitochondrial H2O2 production under varying O2 tensions. https://doi.org/10.1016/bs.mcb.2019.12.008 | Mouse | Nervous system | Isolated mitochondria | Oxidative stress;RONS | |
| Keeley 2019 Physiol Rev | 2019 | Keeley TP, Mann GE (2019) Defining physiological normoxia for improved translation of cell physiology to animal models and humans. https://doi.org/10.1152/physrev.00041.2017 | |||||
| Ast 2019 Nat Metab | 2019 | Ast T, Mootha VK (2019) Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat Metab 1:858-860. | |||||
| Stepanova 2018 J Neurochem | 2018 | Stepanova A, Konrad C, Manfredi G, Springett R, Ten V, Galkin A (2018) The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem 148:731-45. | Mouse | Nervous system | Isolated mitochondria | Ischemia-reperfusion Oxidative stress;RONS | |
| Stuart 2018 Oxid Med Cell Longev | 2018 | Stuart JA, Fonseca JF, Moradi F, Cunningham C, Seliman B, Worsfold CR, Dolan S, Abando J, Maddalena LA (2018) How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid Med Cell Longev 2018:8238459. | |||||
| Stepanova 2018 J Cereb Blood Flow Metab | 2018 | Stepanova A, Konrad C, Guerrero-Castillo S, Manfredi G, Vannucci S, Arnold S, Galkin A (2018) Deactivation of mitochondrial complex I after hypoxia-ischemia in the immature brain. J Cereb Blood Flow Metab 39:1790-802. | Rat | Nervous system | Isolated mitochondria | Hypoxia Ischemia-reperfusion | |
| Stepanova 2017 J Cereb Blood Flow Metab | 2017 | Stepanova A, Kahl A, Konrad C, Ten V, Starkov AS, Galkin A (2017) Reverse electron transfer results in a loss of flavin from mitochondrial complex I: Potential mechanism for brain ischemia-reperfusion injury. J Cereb Blood Flow Metab 37:3649-58. | Mouse | Nervous system | Isolated mitochondria | Ischemia-reperfusion | |
| Harrison 2015 J Appl Physiol | 2015 | Harrison DK, Fasching M, Fontana-Ayoub M, Gnaiger E (2015) Cytochrome redox states and respiratory control in mouse and beef heart mitochondria at steady-state levels of hypoxia. J Appl Physiol 119:1210-8. https://doi.org/10.1152/japplphysiol.00146.2015 | Mouse Bovines | Heart | Isolated mitochondria | Hypoxia | |
| Carreau 2011 J Cell Mol Med | 2011 | Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. https://doi.org/10.1111/j.1582-4934.2011.01258.x | |||||
| Richardson 2006 J Physiol | 2006 | Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. https://doi.org/10.1113/jphysiol.2005.102327 | Human | Skeletal muscle | Hypoxia | ||
| Pettersen 2005 Cell Prolif | 2005 | Pettersen EO, Larsen LH, Ramsing NB, Ebbesen P (2005) Pericellular oxygen depletion during ordinary tissue culturing, measured with oxygen microsensors. Cell Prolif 38:257-67. | |||||
| Gnaiger 2003 Adv Exp Med Biol | 2003 | Gnaiger E (2003) Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. https://doi.org/10.1007/978-1-4419-8997-0_4 | Human Rat | Heart Liver Endothelial;epithelial;mesothelial cell Fibroblast | Intact cells Permeabilized cells Permeabilized tissue Isolated mitochondria Oxidase;biochemical oxidation | ||
| Gnaiger 2001 Respir Physiol | 2001 | Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. https://doi.org/10.1016/S0034-5687(01)00307-3 | Human Rat | Heart Liver Endothelial;epithelial;mesothelial cell HUVEC | Intact cells Isolated mitochondria | Oxidative stress;RONS | |
| Gnaiger 2000 Proc Natl Acad Sci U S A | 2000 | Gnaiger E, Méndez G, Hand SC (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A 97:11080-5. https://doi.org/10.1073/pnas.97.20.11080 | Rat Artemia Crustaceans | Liver | Isolated mitochondria | ||
| Gnaiger 1998 J Exp Biol | 1998 | Gnaiger E, Lassnig B, Kuznetsov AV, Rieger G, Margreiter R (1998) Mitochondrial oxygen affinity, respiratory flux control, and excess capacity of cytochrome c oxidase. https://doi.org/10.1242/jeb.201.8.1129 | Human Rat | Heart Liver Endothelial;epithelial;mesothelial cell HUVEC | Isolated mitochondria Enzyme Oxidase;biochemical oxidation Intact cells | ||
| Gnaiger 1998 Biochim Biophys Acta | 1998 | Gnaiger E, Lassnig B, Kuznetsov AV, Margreiter R (1998) Mitochondrial respiration in the low oxygen environment of the cell: Effect of ADP on oxygen kinetics. Biochim Biophys Acta 1365:249-54. https://doi.org/10.1016/S0005-2728(98)00076-0 | Rat | Heart Liver | Isolated mitochondria | ||
| Gnaiger 1995 J Bioenerg Biomembr | 1995 | Gnaiger E, Steinlechner-Maran R, Méndez G, Eberl T, Margreiter R (1995) Control of mitochondrial and cellular respiration by oxygen. https://doi.org/10.1007/BF02111656 | Human Rat | Liver Endothelial;epithelial;mesothelial cell HUVEC | Isolated mitochondria Intact cells | ||
| Gnaiger 1993 Transitions | 1993 | Gnaiger E (1993) Homeostatic and microxic regulation of respiration in transitions to anaerobic metabolism. In: The vertebrate gas transport cascade: Adaptations to environment and mode of life. Bicudo JEPW (ed), CRC Press, Boca Raton, Ann Arbor, London, Tokyo:358-70. | Reptiles Fishes Crustaceans Annelids | Intact organism | |||
| Gnaiger 1991 Soc Exp Biol Seminar Series | 1991 | Gnaiger E (1991) Animal energetics at very low oxygen: Information from calorimetry and respirometry. In: Strategies for gas exchange and metabolism. Woakes R, Grieshaber M, Bridges CR (eds), Soc Exp Biol Seminar Series 44, Cambridge Univ Press, London:149-71. | Annelids | Intact organism | |||
| Gnaiger 1983 J Exp Zool | 1983 | Gnaiger E (1983) Heat dissipation and energetic efficiency in animal anoxibiosis. Economy contra power. J Exp Zool 228:471-90. | Annelids Molluscs | Skeletal muscle | Intact organism |
- Abstracts: Tissue normoxia
| Year | Reference | Organism | Tissue;cell | Preparations | Stress | Diseases | |
|---|---|---|---|---|---|---|---|
| Donnelly 2022 Abstract Bioblast | 2022 | 2.1. «10+5» Donnelly Chris, Schmitt S, Cecatto C, Cardoso L, Komlodi T, Place N, Kayser B, Gnaiger E (2022) The ABC of hypoxia – what is the norm. Bioblast 2022: BEC Inaugural Conference. In: https://doi.org/10.26124/bec:2022-0001 »MitoFit Preprint« | Oxidative stress;RONS Hypoxia | ||||
| Gnaiger 2018 AussieMit | 2018 | Komlodi Timea, Sobotka Ondrej, Doerrier Carolina, Gnaiger Erich (2018) Mitochondrial H2O2 production is low under tissue normoxia but high at in-vitro air-level oxygen pressure - comparison of LEAK and OXPHOS states. AussieMit 2018 Melbourne AU. | Mouse Saccharomyces cerevisiae | Heart Nervous system | Isolated mitochondria Intact cells | Oxidative stress;RONS Hypoxia | |
| Sobotka 2018 MiP2018 | 2018 | Measurement of ROS production under hypoxia and unexpected methodological pitfalls of Amplex UltraRed assay. | Mouse Saccharomyces cerevisiae | Heart Nervous system | Isolated mitochondria | Hypoxia | |
| Komlodi 2017 MiP2017 | 2017 | H2O2 production under hypoxia in brain and heart mitochondria: does O2 concentration matter? | Mouse | Heart Nervous system | Isolated mitochondria | Oxidative stress;RONS Hypoxia |
Cited by
- 91 articles in PubMed (2024-04-03) https://pubmed.ncbi.nlm.nih.gov/11718759/
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Cardoso et al (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. doi:10.26124/bec:2021-0001
- Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003
- Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi:10.26124/BEC:2021-0004
- Komlódi T, Schmitt S, Zdrazilova L, Donnelly C, Zischka H, Gnaiger E. Oxygen dependence of hydrogen peroxide production in isolated mitochondria and permeabilized cells. MitoFit Preprints (in prep).
- Komlodi et al (2022) Hydrogen peroxide production, mitochondrial membrane potential and the coenzyme Q redox state measured at tissue normoxia and experimental hyperoxia in heart mitochondria. MitoFit Preprints 2021 (in prep)
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
Labels: MiParea: Respiration, Instruments;methods, Comparative MiP;environmental MiP
Stress:Oxidative stress;RONS Organism: Human, Rat Tissue;cell: Heart, Liver, Endothelial;epithelial;mesothelial cell, HUVEC Preparation: Isolated mitochondria, Intact cells
Regulation: ADP, Coupling efficiency;uncoupling, Oxygen kinetics, Threshold;excess capacity Coupling state: OXPHOS
HRR: Oxygraph-2k, TIP2k
ATP, Steady state, Tissue normoxia, BEC 2020.1, BEC 2020.2, MitoFit 2021 MgG, MitoFit 2021 CoQ, MitoFit 2021 AmR-O2, MitoFit 2021 AmR, MitoFit 2021 Tissue normoxia, MitoFit 2021 BCA, BEC2021.5, PLoSONE2022ace-sce, MitoFit2022Hypoxia, MitoFit2022QC