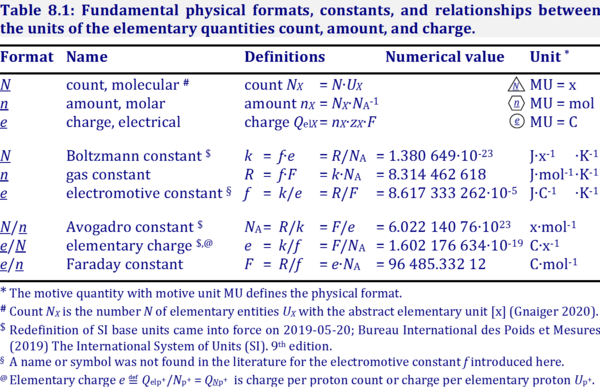
Description
Iconic symbols are used in ergodynamics to indicate more explicitely — compared to standard SI or IUPAC symbols — the quantity represented and some boundary conditions. This is particularly the case in normalized quantities (ratios of quantities). Iconic (or canonical) symbols help to clarify the meaning, are based on SI and IUPAC symbols as far as possible, and may be translated into more commonly used, practical symbols. Several ambiguities in SI and IUPAC symbols are eliminated by the systematic structure of iconic symbols, but it may be impossible to avoid all ambiguities, particulary when long (canonical) symbols are abbreviated in a particular context. Clarity is improved always by showing the unit of a quantity together with the symbol of the quantity. Iconic symbols cannot be identical with IUPAC symbols when a different definition is used — this would add to the confusion. For example, the IUPAC symbols nB [mol] and VB [m3] denote amount and volume of B. Consequently, it should be expected, that the symbol QB indicates charge of B [C]. However, the IUPAC symbol QB is used for particle charge per ion B [C·x-1]. This prohibits a consistent definition of QB as a potential iconic symbol for charge carried by a given quantity of ions B with unit [C], instead of particle charge per ion B with unit [C·x-1]. Hence, the conventional ambigous system forces compatible iconic symbols to be more complicated, using QelB [C] and QNB [C·x-1] to distinguish charge of B from charge per elementary B. QnB [C·mol-1] is charge per molar amount of B.
Reference: Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E (2020-11-30) last update 2022-10-19
- The use of SI and IUPAC symbols and units is recommended for clarity and consistency of presenting concepts and reporting data, as far as consistency and disambiguation are implemented in these international conventions.
What do iconic symbols tell us?
How did Peter Mitchell coin Δp ?
- Mitchell (1966) introduced the symbol Δp for the protonmotive force. Peter Mitchell received the Nobel Price in Chemistry in 1987 for the underlying concept of the pmF. The symbol Δp is unrelated to the symbol ΔpH — the symbol pH stems from the term potentia hydrogenii. What is the message of Δp (Gnaiger 2020)?
- ΔmFeH+ [V] is the iconic symbol for protonmotive force in the electrical format with unit [V = J·C-1]. Starting from the proton and going clockwise, this symbol can be read as "H+ e m Δ F" or "hydrogen ion \ in electrical format \ motive \ difference \ force", in brief: protonmotive force pmF. If you see the symbol Δp as frequently used in bioenergetics, the only iconic aspect is the Δ indicating a difference, whereas the entire meaning of Δp has to be explained to any outsider. Outsiders are insiders in other fields: Δp [Pa] is a pressure difference in respiratory medicine and cardiovascular physiology, is a death penalty for professional and other high-risk divers, and is generally a pressure difference in fluid dynamics and the ideal gas equation Δp = Δn·V-1·RT. Why are we content with using a symbol for pressure when expressing the protonmotive force? To remove this aspect of ambiguity (= double meaning), Michtell's classical Δp for the pmF should be maintained instead of the italicized Δp, to read 'p' as the IUPAC symbol for the proton, instead of "p" for pressure. When Peter Mitchell used a typewriter for his Grey Book (1966), italic versus upright font was not a practical option — using underline in place of italic is tedious. Today, we should use the traditional symbol Δp for the pmF with reference to the proton, and Δp for a pressure difference. Or do iconic symbols have a clear advantage in general communication?
Molar mass and molar volume
- MB is the IUPAC symbol for molar mass of entity B, which is defined for a pure substance as MB = m/nB [kg·mol-1]. Subscript B is ambiguous, indicating "per mole B" in MB, but "of type B" in nB.
- Subscript B in VB does not imply the meaning 'molar volume' [m3·mol-1] (comparable to molar mass MB [kg·mol-1]), but VB [m3] is the volume of a given quantity of entities B (comparable with nB). Therefore, IUPAC uses the ad hoc-symbol Vm,B [m3·mol-1] for molar volume of entity B (but not Mm,B for molar mass).
- Due to the ambiguity of elements in these symbols and an inconsistent system of symbols, we are forced to memorize these IUPAC symbols like complex Chinese characters. In contrast, iconic symbols are composed of readable letters that carry consistent and visible — iconic — meaning. Iconic symbols follow a visible structure, as far as possible. Once you learn the 'iconic alphabet', you can read a variety of symbols like words composed of letters, instead of having to learn and memorize each complex symbol separately. Did Chinese culture exert such an impact on IUPAC, and even more so on all scientists using non-iconic symbols? (This does not imply, that the ~50 000 characters in a standard national Chinese dictionary lack any iconic traces.) The following table illustrates the systematic principle of iconic compared to IUPAC symbols:
- Table: Mass and volume — molar mass and molar volume: Iconic symbols show the quantity, the format n of the normalization in the subscript, and the entity type B in the subscript. The normalized quantities are per B. In the quantities mB, nB, and VB, the subscript B without attachment to a format indicates the quantity of B. Molar count [x·mol-1] and molar charge [C·mol-1] can be added to the list. The corresponding iconic symbols (NnB = NB/nB and QnB = QelB/nB) follow suit, but their counterparts of IUPAC symbols (NA and zB·F), are far from helping to comprehend the underlying concept.
Quantity Unit Normalized for amount Unit Iconic symbol Unit IUPAC symbol Quantity mass mB [kg] / amount nB [mol] = mnB [kg·mol-1] MB molar mass volume VB [m3] / amount nB [mol] = VnB [m3·mol-1] Vm,B molar volume count NB [x] / amount nB [mol] = NnB [x·mol-1] NA molar count; Avogadro constant charge QelB [C] / amount nB [mol] = QnB [C·mol-1] zB·F molar charge; zB · Faraday constant
Abbreviation of iconic symbols
- Exmaple
- The symbol ΔmFH+ [kJ·MU-1] for the pmF uses the subscript 'm' to define the partial transformations as m = d+el, accounting for the two partial transformations of chemical diffusion d and electric el. This excludes, therefore, implicitly other possible energy transformations, such as changes in temperature dT or barometric pressure dp.
- On the other hand, in ΔmFH+ the contributions of d and el are not distinguished, which requires explicit separation of the partial contributions as ΔdFH+ (specific for H+) and ΔelFp+ (not specific for H+ but general for protons or positive charge p+). The symbols ΔmFH+, ΔdFH+, and ΔelFp+ do not specify the motive unit [MU].
- The symbol ΔmFnH+ [kJ·mol-1] for the pmF includes the specification of the molar format n. If all quantities are expressed in the molar format in a particular context, then the symbol may be abbreviated as ΔmFH+ [kJ·mol-1]. This opens up an ambiguity, since in another context the same abbreviated symbol ΔmFH+ would indicate the pmF in the electrical format e with electrical units [V].
- Even the symbol ΔmFnH+ is ambiguous, since the compartmental direction of the pmF is not specified. A positive or negative numerical value of ΔmFnH+, therefore, cannot be interpreted without further specification. This is achieved by canonical extension of the iconic symbol as ΔmFnH+pos, which indicates the direction from the negative compartment (mt-matrix) to the positive compartment across the mtIM.
- Exmaple
- Abbreviation of iconic symbols in a well-defined context improves readability and shortens impractically long equations.
Normalized quantities
- Exmaples
- protonmotive force ΔmFeH+ = pmF [V]
- proton charge QNH+ = e [C·x-1]
- particle charge QNX [C·x-1]
- Exmaples
- Iconic symbols show the quantity, the format of the normalization in the subscript (N, n, e), and the entity specified in the subscript (X). The normalized quantities are per X. In the quantities QelX, NX, nX, VX, mX, the subscript X without attachment to a format indicates the quantity of X.
Canonical comments on IUPAC definitions in the context of charge
Charge of the proton versus charge per proton
- Proton charge is the elementary charge e [C·x-1], which is charge per count of protons.
Qel ≝ Qelp+ [C]
e ≝ QNp+ = Qel·Np+-1 [C∙x-1]
- The distinction of charge of particles versus charge per single particle is not made sufficiently clear by IUPAC, when defining "-e is the charge of an electron" — it must be corrected to "-e is the charge per electron".
- For comparison, the name "charge density of electrons" is used by IUPAC with symbol ρ [C·m-3]. Dividing ρ by the count concentration of electrons [x·m-3], we obtain the unit [C·x-1] for the electron charge. Therefore, electron charge (or proton charge) is clearly the charge per particle.
Ambiguity of QB
- IUPAC (Cohen 2008 IUPAC Green Book) defines the charge number as
IUPAC: zB = QB·e-1
- Therefore, QB = zB∙e. The subscript in QB indicates per elementary entity B. This is opposite to the subscript in VB as the symbol for the volume of a substance of type B (e.g. VO2 [L]). For consistency with this convention, the symbol QelB or QelX [C] is used for indicating charge of a substance of type B or X, distinguished from particle charge as the quantity of charge per elementary entity X with symbol QNX [C∙x-1]. To avoid too long and multiple subscript levels, QNX is used instead of QUX, and the ‘el’ is dropped from QelNX. The particle charge QNH+ per hydrogen ion is identical to the definition of the elementary charge e. Therefore, the charge number of the hydrogen ion is zH+ = QNH+/e = 1. In summary:
zB = QNB·e-1
QNB = QelB·NB-1 [C∙x-1]
Keywords
- » charge QelX
- » charge number zX
- » electrochemical constant f
- » elementary charge e
- » Faraday constant F
- » hydrogen ion versus proton
- » iconic symbols
- » motive entity
- » particle charge QNX
- Bioblast links: Charge - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Normalization of charge and iconic symbols
- Iconic symbols show the quantity, the format of the normalization in the subscript (N, n, e), and the entity specified in the subscript (X). The normalized quantities are per X. In the quantities QelX, NX, nX, VX, mX, the subscript X without attachment to a format indicates the quantity of X.
Quantity Unit Normalized for quantity Unit Iconic symbol Unit Practical symbol Quantity charge QelX [C] / count NX [x] = QNX [C·x-1] particle charge (IUPAC: QB) charge QelX [C] / amount nX [mol] = QnX [C·mol-1] charge number times Faraday constant charge QelX [C] / volume VX [m3] = QVX [C·m-3] ρel charge density charge QelX [C] / mass mX [kg] = QmX [C·kg-1] specific charge count NX [x] / charge QelX [C] = NeX [x·C-1] amount nX [mol] / charge QelX [C] = neX [mol·C-1] volume VX [m3] / charge QelX [C] = VeX [m3·C-1] ρel-1 mass mX [kg] / charge QelX [C] = meX [kg·C-1]
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Bioblast link«
- Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. - »Bioblast link«
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. - »Bioblast link«
- Gnaiger E (2021) The elementary unit — canonical reviewer's comments on: Bureau International des Poids et Mesures (2019) The International System of Units (SI) 9th ed. MitoFit Preprints 2020.4.v2. https://doi.org/10.26124/mitofit:200004.v2.
- Grosholz ER (2007) Representation and productive ambiguity in mathematics and the sciences. Oxford Univ Press:312 pp. - »Bioblast link«
- Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenergetics 1807 (2011):1507-38. - »Bioblast link« – The Grey Book
MitoPedia concepts:
Ergodynamics