| Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi.org/10.26124/bec:2021-0004 |
» Bioenerg Commun 2021.4. ![]() published online 2021-12-21
published online 2021-12-21
Komlodi Timea, Sobotka Ondrej, Gnaiger Erich (2021) Bioenerg Commun
Abstract: ![]() doi:10.26124/bec:2021-0004
doi:10.26124/bec:2021-0004
The fluorometric Amplex UltraRed AmR assay is frequently used for quantitative assessment of hydrogen peroxide production. It is specific to H2O2, can be calibrated accurately, and allows continuous real-time measurement. Without correction for the background fluorescence slope, however, H2O2-independent formation of the fluorescent product UltroxRed (or resorufin) leads to artefacts.
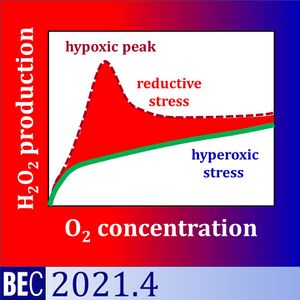
We analysed (1) the medium specificity of the background fluorescence slope of the AmR assay, and (2) the oxygen dependence of H2O2 flux in baker´s yeast Saccharomyces cerevisiae. Apparent H2O2 flux, O2 concentration and O2 flux were measured simultaneously by high-resolution respirometry equipped with the fluorescence module. The apparent H2O2 flux of yeast showed a maximum under hypoxia when incubated in Dulbecco´s Phosphate Buffered Saline DPBS or KCl-medium. This hypoxic peak increased with the sequential number of normoxic-anoxic transitions. Even in the absence of yeast, the fluorescence slope increased at low O2 levels as a function of fluorescence intensity. The hypoxic peak was not observed in mitochondrial respiration medium MiR05.
Therefore, the hypoxic peak was a medium-specific background effect unrelated to cell physiology. In MiR05, H2O2 production of yeast decreased linearly from hyperoxia to hypoxia, with a steep decline towards anoxia. Respiration and oxygen dependence expressed as p50 of yeast were higher in MiR05 than DPBS. Respiration was a hyperbolic function of oxygen concentration in the low-oxygen range. The flux-dependence of oxygen affinity explained the higher p50 in MiR05.
• Keywords: Amplex UltraRed, AmR, hydrogen peroxide production, H2O2 flux, respiration media, mitochondrial respiration medium, MiR05, oxygen dependence, yeast, reductive stress, anoxia, hypoxia, O2 kinetics, respiration, reoxygenation
• Bioblast editor: Gnaiger E
• O2k-Network Lab: AT Innsbruck Oroboros
ORCID: ![]() Komlodi T,
Komlodi T,
![]() Sobotka O
Sobotka O
![]() Gnaiger E
Gnaiger E
Data availability
- Original files are available Open Access at Zenodo repository: 10.5281/zenodo.5785626
Support
- Supported by project NextGen-O2k which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 859770. Ondrej Sobotka ́s secondments were founded by PROGRES Q40/02.
References
| Link | Reference | Year | View |
|---|---|---|---|
| Aon 2010 Biochim Biophys Acta | Aon MA, Cortassa S, O'Rourke B (2010) Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta 1797:865-77. | 2010 | PMID:20175987 Open Access |
| Bienert 2006 Biochim Biophys Acta | Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994-1003. | 2006 | PMID:16566894 Open Access |
| Boveris 1973 Biochem J | Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707-16. | 1973 | PMID: 4749271 Open Access |
| Brand 2016 Free Radical Biol Med | Brand MD (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radical Biol Med 100:14-31. | 2016 | PMID: 27085844 Open Access |
| Buettner 2013 Cell Biochem Biophys | Buettner GR, Wagner BA, Rodgers VG (2013) Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem Biophys 67:477-83. | 2013 | Open access |
| Chandel 1998 Proc Natl Acad Sci | Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci 95:11715-20. | 1998 | PMID:9751731 Open Access |
| Crowe 1998 Annu Rev Physiol | Crowe JH, Carpenter JF, Crowe LM (1998) The role of vitrification in anhydrobiosis. Annu Rev Physiol 60:73-103. | 1998 | PMID:9558455 |
| Dawson 1993 Am J Physiol | Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ (1993) Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol 264:C961-7. | 1993 | PMID:8386454 |
| Dikalov 2014 Antioxid Redox Signal | Dikalov SI, Harrison DG (2014) Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 20:372–82. | 2014 | PMID: 22978713 Open Access |
| Doerrier 2018 Methods Mol Biol | Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782:31-70. https://doi.org/10.1007/978-1-4939-7831-1_3 | 2018 | PMID: 29850993 » |
| Duong 2020 Mitochondrion | Duong QV, Hoffman A, Zhong K, Dessinger MJ, Zhang Y, Bazil JN (2020) Calcium overload decreases net free radical emission in cardiac mitochondria. Mitochondrion 51:126-39. | 2020 | PMID: 31982614 |
| Dutton 1989 Arch Biochem Biophys | Dutton DR, Reed GA, Parkinson A (1989) Redox cycling of resorufin catalyzed by rat liver microsomal NADPH-cytochrome P450 reductase. Arch Biochem Biophys 268:605-16. | 1989 | PMID:2464338 |
| Dębski 2016 Free Radic Biol Med | Dębski D, Smulik R, Zielonka J, Michałowski B, Jakubowska M, Dębowska K, Adamus J, Marcinek A, Kalyanaraman B, Sikora A (2016) Mechanism of oxidative conversion of Amplex® Red to resorufin: pulse radiolysis and enzymatic studies. Free Radic Biol Med 95:323-32. | 2016 | PMID:27021961 Open Access |
| Gnaiger 2001 Respir Physiol | Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. https://doi.org/10.1016/S0034-5687(01)00307-3 | 2001 | Respir Physiol 128:277-97. PMID: 11718759 |
| Gnaiger 2003 Adv Exp Med Biol | Gnaiger E (2003) Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. https://doi.org/10.1007/978-1-4419-8997-0_4 | 2003 | Adv Exp Med Biol 543:39-55. PMID: 14713113 |
| Gnaiger 2008 POS | Gnaiger E (2008) Polarographic oxygen sensors, the oxygraph and high-resolution respirometry to assess mitochondrial function. In: Mitochondrial dysfunction in drug-induced toxicity (Dykens JA, Will Y, eds) John Wiley & Sons, Inc, Hoboken, NJ:327-52. | 2008 | |
| Gnaiger 2000 Life in the Cold | Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R (2000) Mitochondria in the cold. In: Life in the Cold (Heldmaier G, Klingenspor M, eds) Springer, Berlin, Heidelberg:431-42. https://doi.org/10.1007/978-3-662-04162-8_45 | 2000 | |
| Gnaiger 1995 J Bioenerg Biomembr | Gnaiger E, Steinlechner-Maran R, Méndez G, Eberl T, Margreiter R (1995) Control of mitochondrial and cellular respiration by oxygen. https://doi.org/10.1007/BF02111656 | 1995 | J Bioenerg Biomembr 27:583-96. PMID: 8746845 |
| Grivennikova 2018 Redox Biol | Grivennikova VG, Kareyeva AV, Vinogradov AD (2018) Oxygen-dependence of mitochondrial ROS production as detected by Amplex Red assay. Redox Biol 17:192-9. | 2018 | PMID:29702406 Open Access |
| Guzy 2007 Antioxid Redox Signal | Guzy RD, Mack MM, Schumacker PT (2007) Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal 9:1317-28. | 2007 | PMID:17627464 |
| Hernansanz-Agustín 2014 Free Radic Biol Med | Hernansanz-Agustin P, Izquierdo-Álvarez A, Sánchez-Gómez FJ, Ramos E, Villa-Piña T, Lamas S, Bogdanova A, Martínez-Ruiz A (2014) Acute hypoxia produces a superoxide burst in cells. Free Radic Biol Med 71:146-56. | 2014 | PMID: 24637263 |
| Hoffman 2007 Am J Physiol Heart Circ Physiol | Hoffman DL, Salter JD, Brookes PS (2007) Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol 292:H101-8. | 2007 | PMID:16963616 Open Access |
| Kalyanaraman 2012 Free Radical Biol Med | Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd, Ischiropoulos H (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biol Med 52:1-6. | 2012 | PMID:22027063 Open Access |
| Koga 1966 Biophys J | Koga S, Echigo A, Nunomura K (1966) Physical properties of cell water in partially dried Saccharomyces cerevisiae.. Biophys J 6:665-74. | 1966 | PMID:5970569 Open Access |
| Komlodi 2018 Methods Mol Biol | Komlodi T, Sobotka O, Krumschnabel G, Bezuidenhout N, Hiller E, Doerrier C, Gnaiger E (2018) Comparison of mitochondrial incubation media for measurement of respiration and hydrogen peroxide production. Methods Mol Biol 1782:137-55. | 2018 | PMID:29850998 |
| Korge 2015 Biochim Biophys Acta | Korge P, Calmettes G, Weiss JN (2015) Increased reactive oxygen species production during reductive stress: the roles of mitochondrial glutathione and thioredoxin reductases. Biochim Biophys Acta 1847:514–25. | 2015 | PMID:25701705 Open Access |
| Krumschnabel 2015 Methods Mol Biol | Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, Gnaiger E (2015) Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol 1264:245-61. | 2015 | PMID: 25631019 |
| Li Puma 2020 Am J Physiol Regul Integr Comp Physiol | Li Puma LC, Hedges M, Heckman JM, Mathias AB, Engstrom MR, Brown AB, Chicco AJ (2020) Experimental oxygen concentration influences rates of mitochondrial hydrogen peroxide release from cardiac and skeletal muscle preparations. Am J Physiol Regul Integr Comp Physiol 318:972-80. | 2020 | PMID: 32233925 » |
| Longo 1996 J Biol Chem | Longo VD, Gralla EB, Valentine JS (1996) Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem 271:12275-80. | 1996 | PMID: 8647826 Open Access |
| Makrecka-Kuka 2015 Biomolecules | Makrecka-Kuka M, Krumschnabel G, Gnaiger E (2015) High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. https://doi.org/10.3390/biom5031319 | 2015 | Biomolecules 5:1319-38. PMID: 26131977 Open Access |
| Mishin 2010 Free Radic Biol Med | Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD (2010) Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic Biol Med 48:1485-91. | 2010 | PMID:20188819 Open Access |
| Miwa 2015 Free Radic Biol Med | Miwa S, Treumann A, Bell A, Vistoli G, Nelson G, Hay S, von Zglinicki T (2015) Carboxylesterase converts Amplex red to resorufin: Implications for mitochondrial H2O2 release assays. Free Radic Biol Med 90:173-83. | 2015 | PMID: 26577176 Open Access |
| Mohanty 1997 J Immunol Methods | Mohanty JG, Jaffe JS, Schulman ES, Raible DG (1997) A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Meth 202:133-41. | 1997 | PMID: 9107302 |
| Ottolenghi 2020 Minerva Anestesiol | Ottolenghi S, Sabbatini G, Brizzolari A, Samaja M, Chiumello D (2020) Hyperoxia and oxidative stress in anesthesia and critical care medicine. Minerva Anestesiol 86:64-75. doi: 10.23736/S0375-9393.19.13906-5 | 2020 | PMID: 31680497 Open Access |
| Paniker 1970 Biochim Biophys Acta | Paniker NV, Srivastava SK, Beutler E (1970) Glutathione metabolism of the red cells. Effect of glutathione reductase deficiency on the stimulation of hexose monophosphate shunt under oxidative stress. Biochim Biophys Acta 215:456-60. | 1970 | PMID:5507367 |
| Piwonski 2012 Proc Natl Acad Sci U S A | Piwonski HM, Goomanovsky M, Bensimon D, Horovitz A, Haran G (2012) Allosteric inhibition of individual enzyme molecules trapped in lipid vesicles. Proc Natl Acad Sci U S A 109:E1437-43. | 2012 | PMID:22562794 Open Access |
| Robb 2018 J Biol Chem | Robb EL, Hall AR, Prime TA, Eaton S, Szibor M, Viscomi C, James AM, Murphy MP (2018) Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 293:9869-79. https://doi.org/10.1074/jbc.RA118.003647 | 2018 | PMID: 29743240 Open Access |
| Scandurra 2010 Adv Exp Med Biol | Scandurra FM, Gnaiger E (2010) Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. https://doi.org/10.1007/978-1-4419-1241-1_2 | 2010 | Adv Exp Med Biol 662:7-25. PMID: 20204766 Open Access |
| Sies 1997 Exp Physiol | Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291-5. | 1997 | PMID:9129943 Open Access |
| Sies 2020 Nat Rev Mol Cell Biol | Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21:363-83. | 2020 | PMID:32231263 |
| Skulachev 1996 Q Rev Biophys | Skulachev VP (1996) Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys 29:169-202. | 1996 | PMID:8870073 |
| Smith 2017 Redox Biol | Smith KA, Waypa GB, Schumacker PT (2017) Redox signaling during hypoxia in mammalian cells. Redox Biol 13:228-34. | 2017 | PMID:28595160 Open Access |
| Stepanova 2020 Methods Cell Biol | Stepanova A, Galkin A (2020) Measurement of mitochondrial H2O2 production under varying O2 tensions. https://doi.org/10.1016/bs.mcb.2019.12.008 | 2020 | Methods Cell Biol 155:273-93. PMID: 32183962 |
| Stepanova 2017 J Cereb Blood Flow Metab | Stepanova A, Kahl A, Konrad C, Ten V, Starkov AS, Galkin A (2017) Reverse electron transfer results in a loss of flavin from mitochondrial complex I: Potential mechanism for brain ischemia-reperfusion injury. J Cereb Blood Flow Metab 37:3649-58. | 2017 | PMID: 28914132 |
| Stepanova 2018 J Cereb Blood Flow Metab | Stepanova A, Konrad C, Guerrero-Castillo S, Manfredi G, Vannucci S, Arnold S, Galkin A (2018) Deactivation of mitochondrial complex I after hypoxia-ischemia in the immature brain. J Cereb Blood Flow Metab 39:1790-802. | 2018 | PMID: 29629602 |
| Stepanova 2018 J Neurochem | Stepanova A, Konrad C, Manfredi G, Springett R, Ten V, Galkin A (2018) The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem 148:731-45. | 2018 | PMID: 30582748 |
| Szibor 2020 J Cell Mol Med | Szibor M, Schreckenberg R, Gizatullina Z, Dufour E, Wiesnet M, Dhandapani PK, Debska-Vielhaber G, Heidler J, Wittig I, Nyman TA, Gaertner U, Hall AR, Pell V, Viscomi C, Krieg T, Murphy MP, Braun T, Gellerich FN, Schlueter KD, Jacobs HT(2020) Respiratory chain signalling is essential for adaptive remodelling following cardiac ischaemia. J Cell Mol Med 24:3534-48. | 2020 | PMID: 32040259 Open Access » |
| Tretter 2014 Methods Enzymol | Tretter L, Ambrus A (2014) Measurement of ROS homeostasis in isolated mitochondria. Methods Enzymol 547:199-223. | 2014 | PMID:25416360 |
| Verkhovsky 1996 Nature | Verkhovsky MI, Morgan JE, Puustein A, Wikström M (1996) Kinetic trapping of oxygen in cell respiration. Nature 380:268-70. | 1996 | PMID:8637579 |
| Votyakova 2004 Arch Biochem Biophys | Votyakova TV, Reynolds IJ (2004) Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys 431:138-44. | 2004 | PMID:15464736 |
| Waypa 2001 Circ Res | Waypa GB, Chandel NS, Schumacker PT (2001) Model for Hypoxic Pulmonary Vasoconstriction Involving Mitochondrial Oxygen Sensing. Circ Res 88:1259–1266. | 2001 | PMID:11420302 Open Access |
| Xiao 2020 Antioxid Redox Signal | Xiao W, Loscalzo J (2020) Metabolic responses to reductive stress. Antioxid Redox Signal 32:1330-47. DOI: 10.1089/ars.2019.7803 | 2020 | PMID:31218894 Open Access |
| Zhao 2011 Free Radic Biol Med | Zhao B, Ranguelova K, Jiang J, Mason RP (2011) Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex Red. Free Radic Biol Med 51:153-9 | 2011 | PMID: 22576106 Open Access |
| Zhao 2012 Free Radic Biol Med | Zhao B, Summers FA, Mason RP (2012) Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Free Radical Biol Med 51: 153–9. | 2012 | PMID:22765927 Open Access |
| Zhou 1997 Anal Biochem | Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253:162-8. | 1997 | PMID:9367498 Open access |
Cited by
- Gnaiger E (2024) Addressing the ambiguity crisis in bioenergetics and thermodynamics. MitoFit Preprints 2024.3. https://doi.org/10.26124/mitofit:2024-0003
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
- Komlódi T, Schmitt S, Zdrazilova L, Donnelly C, Zischka H, Gnaiger E. Oxygen dependence of hydrogen peroxide production in isolated mitochondria and permeabilized cells. MitoFit Preprints (in prep).
- Komlódi T, Gnaiger E (2022) Discrepancy on oxygen dependence of mitochondrial ROS production - review. MitoFit Preprints 2022 (in prep).
- Komlodi et al (2022) Hydrogen peroxide production, mitochondrial membrane potential and the coenzyme Q redox state measured at tissue normoxia and experimental hyperoxia in heart mitochondria. MitoFit Preprints 2021 (in prep)
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
Preprint
Labels: MiParea: Respiration, Instruments;methods
Stress:Oxidative stress;RONS, Hypoxia Organism: Saccharomyces cerevisiae Tissue;cell: Other cell lines Preparation: Intact cells
Regulation: Oxygen kinetics Coupling state: ROUTINE
HRR: Oxygraph-2k, O2k-Fluorometer, O2k-Protocol
SUIT-013, SUIT-013 AmR ce D023, SUIT-009, SUIT-009 AmR mt D021, SUIT-009 AmR pce D019, SUIT-018, SUIT-018 AmR mt D031, SUIT-006, SUIT-006 AmR mt D048, SUIT-003 AmR ce D017, SUIT-003 AmR ce D058, AmR, BEC2021.5, PLoSONE2022ace-sce, MitoFit2022Hypoxia, MitoFit 2022 NADH, MitoFit 2021 AmR, MitoFit 2022 ROS review, MitoFit 2021 Tissue normoxia, O2k-Demo, O2k-MultiSensor, H2O2, Gnaiger 2024 MitoFit