Lemieux 2017 Sci Rep
| Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. doi:10.1038/s41598-017-02789-8 |
» PMID: 28588260 Sci Rep Open Access
Lemieux Helene, Blier PU, Gnaiger Erich (2017) Sci Rep
Abstract: Fuel substrate supply and oxidative phosphorylation are key determinants of muscle performance. Numerous studies of mammalian mitochondria are carried out (i) with substrate supply that limits electron flow, and (ii) far below physiological temperature. To analyze potentially implicated biases, we studied mitochondrial respiratory control in permeabilized mouse myocardial fibers using high-resolution respirometry. The capacity of oxidative phosphorylation at 37 °C was nearly two-fold higher when fueled by physiological substrate combinations reconstituting tricarboxylic acid cycle function, compared with electron flow measured separately through NADH to Complex I or succinate to Complex II. The relative contribution of the NADH pathway to physiological respiratory capacity increased with a decrease in temperature from 37 to 25 ºC. The apparent excess capacity of cytochrome c oxidase above physiological pathway capacity increased sharply under hypothermia due to limitation by NADH-linked dehydrogenases. This mechanism of mitochondrial respiratory control in the hypothermic mammalian heart is comparable to the pattern in ectotherm species, pointing towards NADH-linked mt-matrix dehydrogenases and the phosphorylation system rather than electron transfer complexes as the primary drivers of thermal sensitivity at low temperature. Delineating the link between stress and remodeling of oxidative phosphorylation is important for understanding metabolic perturbations in disease evolution and cardiac protection.
• Bioblast editor: Gnaiger E • O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Oroboros, CA Rimouski Blier PU, CA Edmonton Lemieux H
Preprints for Gentle Science
- Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of oxidative phosphorylation by temperature in the heart. bioRxiv doi: https://doi.org/10.1101/103457. - »Bioblast link«
![]() In the spirit of COST Action MitoEAGLE WG1
In the spirit of COST Action MitoEAGLE WG1
SUIT protocols
Nomenclature
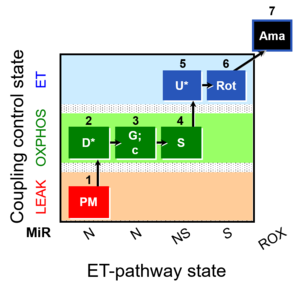
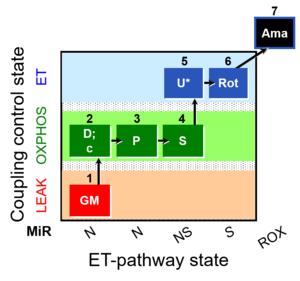
- NADH-linked or N-pathways (CI-entry into Q); succinate-linked or S-pathway (CII-entry into Q); NS-pathway (convergent CI&II-entry into Q)
![]() Contribution to K-Regio MitoFit
Contribution to K-Regio MitoFit
Further references
- Li P, Wang B, Sun F, Li Y, Li Q, Lang H, Zhao Z, Gao P, Zhao Y, Shang Q, Liu D, Zhu Z (2015) Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci Rep 5:10229. - »Bioblast link«
Cited by
- 33 articles in PubMed (2023-11-30) https://pubmed.ncbi.nlm.nih.gov/28588260/
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
- Cardoso et al (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. doi:10.26124/bec:2021-0001
- Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003
Labels: MiParea: Respiration, Comparative MiP;environmental MiP
Organism: Mouse
Tissue;cell: Heart
Preparation: Permeabilized tissue
Enzyme: Marker enzyme
Regulation: Cyt c, Inhibitor, Temperature, Threshold;excess capacity, Uncoupler
Coupling state: LEAK, OXPHOS, ET
Pathway: N, S, NS
HRR: Oxygraph-2k, O2k-Protocol
1PM;2D;3G;4S;5U;6Rot-, SUIT-008, 1GM;2D;3P;4S;5U;6Rot-, SUIT-014, SUIT-008 O2 pce D025, MitoFitPublication, 2017-06, MitoEAGLEPublication, BEC 2020.1, BEC 2020.2, MitoFit 2021 MgG, MitoFit 2021 CoQ