Madamanchi 2007 Circ Res
| Madamanchi NR, Runge MS (2007) Mitochondrial dysfunction in atherosclerosis. Circ Res 100:460-73. doi: 10.1161/01.RES.0000258450.44413.96 |
Madamanchi NR, Runge MS (2007) Circ Res
Abstract: Increased production of reactive oxygen species in mitochondria, accumulation of mitochondrial DNA damage, and progressive respiratory chain dysfunction are associated with atherosclerosis or cardiomyopathy in human investigations and animal models of oxidative stress. Moreover, major precursors of atherosclerosis-hypercholesterolemia, hyperglycemia, hypertriglyceridemia, and even the process of aging-all induce mitochondrial dysfunction. Chronic overproduction of mitochondrial reactive oxygen species leads to destruction of pancreatic beta-cells, increased oxidation of low-density lipoprotein and dysfunction of endothelial cells-factors that promote atherosclerosis. An additional mechanism by which impaired mitochondrial integrity predisposes to clinical manifestations of vascular diseases relates to vascular cell growth. Mitochondrial function is required for normal vascular cell growth and function. Mitochondrial dysfunction can result in apoptosis, favoring plaque rupture. Subclinical episodes of plaque rupture accelerate the progression of hemodynamically significant atherosclerotic lesions. Flow-limiting plaque rupture can result in myocardial infarction, stroke, and ischemic/reperfusion damage. Much of what is known on reactive oxygen species generation and modulation comes from studies in cultured cells and animal models. In this review, we have focused on linking this large body of literature to the clinical syndromes that predispose humans to atherosclerosis and its complications.
• Bioblast editor: Gnaiger E
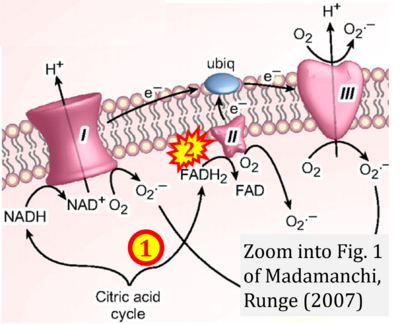
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase