Description
.
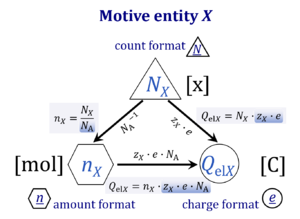
A motive entity Xtr is an entity involved in a transformation including spacial transfer. Motive entities (transformants) are expressed in different motive units [MU] depending on the energy transformation under study and the chosen format. Flows are defined as advancement in terms of stoichiometric motive entities per time. Isomorphic forces are partial derivatives of Gibbs energy per advancement. Ions carrying a positive charge (cations) or negative charge (anions) may be considered as a paradigm of motive entities, since Faraday did not coin but introduced the term 'ion', which is old Greek for 'going' — advancing to the cathode or anode and thus generating an electric current.
Abbreviation: Xtr [MU]
Reference: Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E 2020-12-01
- Bioblast links: Normalization - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Quantities for normalization
- » Count in contrast to Number
- » Mitochondrial marker
- » O2k-Protocols: mitochondrial and marker-enzymes
- » Citrate synthase activity
- Quantities for normalization
- General
- Related keyword lists
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
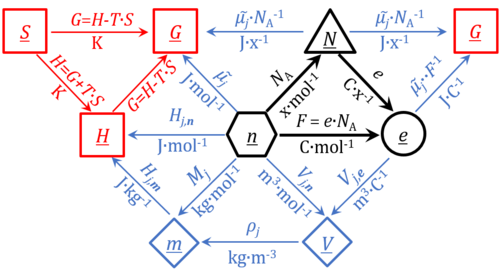
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
MitoPedia concepts:
Ergodynamics