Description
The amount of substance, n, is a base physical quantity, and the corresponding SI unit is the mole [mol]. Amount of substance (sometimes abbreviated as 'amount' or 'chemical amount') is proportional to the number of specified elementary entities of that substance, and the universal proportionality constant is the reciprocal value of the Avogadro constant.
Abbreviation: n
Reference: Cohen 2008 IUPAC Green Book
Communicated by Gnaiger E 2018-11-01
Changes of amount
- The amount of substance i, ni, contained in a system changes due to internal and external transformations,
dni = dini + deni
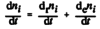
- The change of amount of i in an open system is due to internal formation in chemical transformations, where drni is positive if i is formed as a product of the reaction and the external transfer of substance i, deni (negative if i flows out of the system and appears as a product in the surroundings [1].
References
- Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - »Bioblast link«
MitoPedia concepts: MiP concept, Ergodynamics
MitoPedia topics:
Substrate and metabolite
