Difference between revisions of "Avogadro constant"
From Bioblast
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=''N''<sub>A</sub> | |abbr=''N''<sub>A</sub> | ||
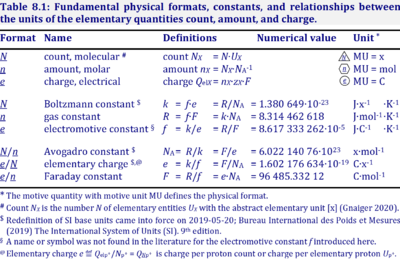
|description=[[File:Table Physical constants.png|left|400px|thumb|]] | |description=[[File:Table Physical constants.png|left|400px|thumb|]] The '''Avogadro constant''', ''N''<sub>A</sub>, has the SI unit [mol<sup>-1</sup>] (IUPAC), but more strictly the units for particles per amount is [x·mol<sup>-1</sup>] (compare [[Elementary charge]]). Therefore, the reciprocal of the Avogadro constant is the proportionality factor between the amount of substance and the number of specified elementary entities of that substance. The Avogadro constant times elementary charge is the [[Faraday constant]]. | ||
|info=[[Gibney 2017 Nature]] | |info=[[Gibney 2017 Nature]] | ||
}} | }} | ||
| Line 7: | Line 7: | ||
|mitopedia concept=Ergodynamics | |mitopedia concept=Ergodynamics | ||
}} | }} | ||
Communicated by [[Gnaiger E]] 2018-10-18 | Communicated by [[Gnaiger E]] 2018-10-18 | ||
Revision as of 12:53, 18 October 2018
Description
The Avogadro constant, NA, has the SI unit [mol-1] (IUPAC), but more strictly the units for particles per amount is [x·mol-1] (compare Elementary charge). Therefore, the reciprocal of the Avogadro constant is the proportionality factor between the amount of substance and the number of specified elementary entities of that substance. The Avogadro constant times elementary charge is the Faraday constant.
Abbreviation: NA
Reference: Gibney 2017 Nature
MitoPedia concepts:
Ergodynamics
Communicated by Gnaiger E 2018-10-18