Thorgersen 2022 Front Microbiol
| Thorgersen MP, Schut GJ, Poole FL 2nd, Haja DK, Putumbaka S, Mycroft HI, de Vries WJ, Adams MWW (2022) Obligately aerobic human gut microbe expresses an oxygen resistant tungsten-containing oxidoreductase for detoxifying gut aldehydes. Front Microbiol 13:965625. https://doi.org/10.3389/fmicb.2022.965625 |
Thorgersen MP, Schut GJ, Poole FL 2nd, Haja DK, Putumbaka S, Mycroft HI, de Vries WJ, Adams MWW (2022) Front Microbiol
Abstract: Brevibacillus massiliensis strain phR is an obligately aerobic microbe that was isolated from human feces. Here, we show that it readily takes up tungsten (W), a metal previously associated only with anaerobes. The W is incorporated into an oxidoreductase enzyme (BmWOR) that was purified from native biomass. BmWOR consists of a single 65 kDa subunit and contains a single W-pyranopterin cofactor and a single [4Fe-4S] cluster. It exhibited high aldehyde-oxidizing activity with very high affinities (apparent Km < 6 μM) for aldehydes common in the human gut and in cooked foods, including furfural, propionaldehyde, benzaldehyde and tolualdehyde, suggesting that BmWOR plays a key role in their detoxification. B. massiliensis converted added furfural to furoic acid when grown in the presence of W, but not in the presence of the analogous element molybdenum. B. massiliensis ferredoxin (BmFd) served as the electron acceptor (apparent Km < 5 μM) for BmWOR suggesting it is the physiological electron carrier. Genome analysis revealed a Fd-dependent rather than NADH-dependent Complex I, suggesting that WOR not only serves a detoxification role but its aldehyde substrates could also serve as a source of energy. BmWOR is the first tungstoenzyme and the first member of the WOR family to be obtained from a strictly aerobic microorganism. Remarkably, BmWOR oxidized furfural in the presence of air (21 % O2, v/v) but only if BmFd was also present. BmWOR is the first characterized member of the Clade 83 WORs, which are predominantly found in extremely halophilic and aerobic archaea (Clade 83A), with many isolated from food sources, while the remaining bacterial members (Clade 83B) include both aerobes and anaerobes. The potential advantages for microbes found in foods and involved in human gut health that harbor O2-resistant WORs, including in Bacillus and Brevibacillus based-probiotics, are discussed.
• Bioblast editor: Gnaiger E
Labels:
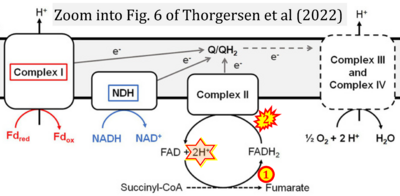
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«