Difference between revisions of "Avogadro constant"
From Bioblast
| Line 10: | Line 10: | ||
:::: In a critical article on dimensionless units in the SI, Mohr and Phillips (2015) comment on the Avogadro constant, ''N''<sub>A</sub>: "''Evidently, this constant can be viewed as the conversion factor between entities and moles.''" The unit mole [mol], however, is the SI base unit for amount of substance, defined by IUPAC (Cohen et al 2008) as: "''Amount of substance is proportional to the number of specified elementary entities of that substance''". Therefore, ''N''<sub>A</sub> is not the conversion factor between entities and moles, but ''N''<sub>A</sub> is the conversion factor between the number of entities and moles of entities. Thus, the unit 'ent' suggested by Mohr and Phillips (2015) for the number of entities would imply, that they should consider 'mol ent' as the unit for amount of substance. In this case, the unit of ''N''<sub>A</sub> should be [ent/(mol ent) = mol<sup>-1</sup>], in line with SI, but contrary to the valid intention of Mohr and Phillips (2015). | :::: In a critical article on dimensionless units in the SI, Mohr and Phillips (2015) comment on the Avogadro constant, ''N''<sub>A</sub>: "''Evidently, this constant can be viewed as the conversion factor between entities and moles.''" The unit mole [mol], however, is the SI base unit for amount of substance, defined by IUPAC (Cohen et al 2008) as: "''Amount of substance is proportional to the number of specified elementary entities of that substance''". Therefore, ''N''<sub>A</sub> is not the conversion factor between entities and moles, but ''N''<sub>A</sub> is the conversion factor between the number of entities and moles of entities. Thus, the unit 'ent' suggested by Mohr and Phillips (2015) for the number of entities would imply, that they should consider 'mol ent' as the unit for amount of substance. In this case, the unit of ''N''<sub>A</sub> should be [ent/(mol ent) = mol<sup>-1</sup>], in line with SI, but contrary to the valid intention of Mohr and Phillips (2015). | ||
:::: ''N''<sub>A</sub> is the conversion factor between | :::: ''N''<sub>A</sub> is the conversion factor between countable entities, expressed in counting units [x], and amount of entities (amount of substance), expressed in moles [mol]. Therefore, the unit of ''N''<sub>A</sub> is 'counting units per mole' [x·mol<sup>-1</sup>]. | ||
Revision as of 23:19, 14 May 2020
Description
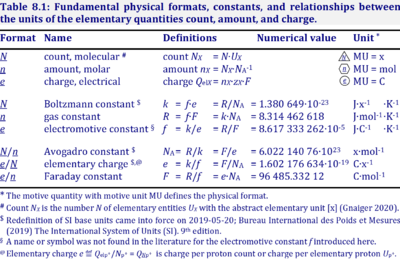
The Avogadro constant, NA, has the SI unit 'per mole' [mol-1] (IUPAC), but more strictly the unit for entities per amount is 'units per mole' [x·mol-1] (compare Elementary charge). Therefore, the reciprocal of the Avogadro constant is the proportionality factor between the amount of substance and the number of specified elementary entities of that substance. The Avogadro constant times elementary charge is the Faraday constant.
Abbreviation: NA [x·mol-1]
Reference: Gibney 2017 Nature
Communicated by Gnaiger Erich 2018-10-18, last update 2020-05-14
Discussion
- In a critical article on dimensionless units in the SI, Mohr and Phillips (2015) comment on the Avogadro constant, NA: "Evidently, this constant can be viewed as the conversion factor between entities and moles." The unit mole [mol], however, is the SI base unit for amount of substance, defined by IUPAC (Cohen et al 2008) as: "Amount of substance is proportional to the number of specified elementary entities of that substance". Therefore, NA is not the conversion factor between entities and moles, but NA is the conversion factor between the number of entities and moles of entities. Thus, the unit 'ent' suggested by Mohr and Phillips (2015) for the number of entities would imply, that they should consider 'mol ent' as the unit for amount of substance. In this case, the unit of NA should be [ent/(mol ent) = mol-1], in line with SI, but contrary to the valid intention of Mohr and Phillips (2015).
- NA is the conversion factor between countable entities, expressed in counting units [x], and amount of entities (amount of substance), expressed in moles [mol]. Therefore, the unit of NA is 'counting units per mole' [x·mol-1].
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Open Access pdf«
- Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. - »Bioblast link«
- Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - »Bioblast link«
- Mohr PJ, Phillips WD (2015) Dimensionless units in the SI. Metrologia 52:40-7. - »Bioblast link«
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
MitoPedia concepts:
Ergodynamics