- high-resolution terminology - matching measurements at high-resolution
MitoPedia: Respiratory states
The MitoPedia terminology is developed continuously in the spirit of Gentle Science.
- Coupling-control states - symbols for rates
- Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1. - »Bioblast link«
- Coupling-control states - symbols for rates
- See also
Respiratory states: recommended terms
| Term | Abbreviation | Description |
|---|---|---|
| Background state | Y | The background state Y (background rate YX) is the non-activated or inhibited respiratory state at background rate, which is low in relation to the higher rate ZX in the reference state Z. The transition from the background state to the reference state is a step change. A metabolic control variable X (substrate, activator) is added to the background state to stimulate flux to the level of the reference state. Alternatively, the metabolic control variable X is an inhibitor, which is present in the background state Y, but absent in the reference state Z. The background state is the baseline of a single step in the definition of the flux control efficiency. In a sequence of step changes, the common baseline state is the state of lowest flux in relation to all steps, which can be used as a baseline correction. |
| Basal respiration | BMR | Basal respiration or basal metabolic rate (BMR) is the minimal rate of metabolism required to support basic body functions, essential for maintenance only. BMR (in humans) is measured at rest 12 to 14 hours after eating in a physically and mentally relaxed state at thermally neutral room temperature. Maintenance energy requirements include mainly the metabolic costs of protein turnover and ion homeostasis. In many aerobic organisms, and particularly well studied in mammals, BMR is fully aerobic, i.e. direct calorimetry (measurement of heat dissipation) and indirect calorimetry (measurement of oxygen consumption multiplied by the oxycaloric equivalent) agree within errors of measurement (Blaxter KL 1962. The energy metabolism of ruminants. Hutchinson, London: 332 pp [1]). In many cultured mammalian cells, aerobic glycolysis contributes to total ATP turnover (Gnaiger and Kemp 1990 [2]), and under these conditions, 'respiration' is not equivalent to 'metabolic rate'. Basal respiration in humans and skeletal muscle mitochondrial function (oxygen kinetics) are correlated (Larsen et al 2011 [3]). » MiPNet article |
| Baseline state | The baseline state in a sequence of step changes is the state of lowest flux in relation to all steps, which can be used as a baseline correction. Correction for residual oxygen consumption, ROX, is an example where ROX is the baseline state. In a single step, the baseline state is equivalent to the background state. | |
| Chemical background | CHB, Chb |  Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. |
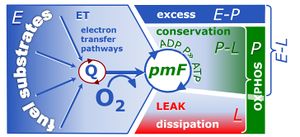
| Coupling-control state | CCS | Coupling-control states are defined in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, homogenates) as LEAK respiration, OXPHOS, and ET states, with corresponding respiration rates (L, P, E) in any electron-transfer-pathway state which is competent for electron transfer. These coupling states are induced by titration of ADP and uncouplers, and application of specific inhibitors of the phosphorylation pathway. In living cells, the coupling-control states are LEAK respiration, ROUTINE, and ET states of respiration with corresponding rates L, R, E, using membrane-permeable inhibitors of the phosphorylation system (e.g. oligomycin) and uncouplers (e.g. CCCP). Coupling-control protocols induce these coupling-control states sequentially at a constant electron-transfer-pathway state. |
| Dyscoupled respiration | Dyscoupled respiration is LEAK respiration distinguished from intrinsically (physiologically) uncoupled and from extrinsic experimentally uncoupled respiration as an indication of extrinsic uncoupling (pathological, toxicological, pharmacological by agents that are not specifically applied to induce uncoupling, but are tested for their potential dyscoupling effect). Dyscoupling indicates a mitochondrial dysfunction. In addition to intrinsic uncoupling, dyscoupling occurs under pathological and toxicological conditions. Thus a distinction is made between physiological uncoupling and pathologically defective dyscoupling in mitochondrial respiration. | |
| E-P excess capacity | E-P | |
| E-R reserve capacity | E-R | |
| ET capacity | E | |
| Electron-transfer-pathway state | ET-pathway state |
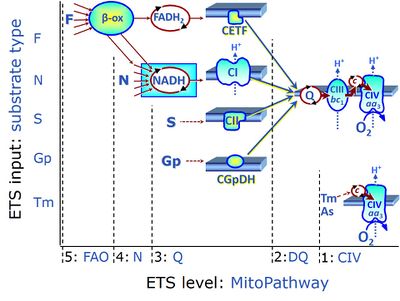
Electron-transfer-pathway states are obtained in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, tissue homogenate) by depletion of endogenous substrates and addition to the mitochondrial respiration medium of fuel substrates (CHNO) activating specific mitochondrial pathways, and possibly inhibitors of specific pathways. Mitochondrial electron-transfer-pathway states have to be defined complementary to mitochondrial coupling-control states. Coupling-control states require ET-pathway competent states, including oxygen supply. Categories of SUIT protocols are defined according to mitochondrial ET-pathway states. » MiPNet article |
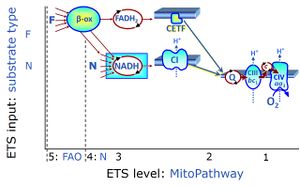
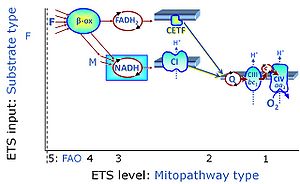
| FN | FN | FN is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state, or CI-linked pathway control) in combination with one or several fatty acids, which are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. FS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| FNS | FNS | FNS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state, or CI-linked pathway control) in combination with succinate (S-pathway control state; S- or CII-linked) and one or several fatty acids, which are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. FNS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| Fatty acid oxidation pathway control state | F, FAO | In the fatty acid oxidation pathway control state (F- or FAO-pathway), one or several fatty acids are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting relative to the N-pathway branch), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). In addition and independent of this source of NADH, the type N substrate malate is required at low concentration (0.1 mM) as a co-substrate for FAO in mt-preparations, since accumulation of Acetyl-CoA inhibits FAO in the absence of malate. Malate is oxidized in a reaction catalyzed by malate dehydrogenase to oxaloacetate (yielding NADH), which then stimulates the entry of Acetyl-CoA into the TCA cycle catalyzed by citrate synthase. Peroxysomal β-oxidation carries out few β-oxidation cycles, thus shortening very-long-chain fatty acids (>C20) for entry into mitochondrial β-oxidation. Oxygen consumption by peroxisomal acyl-CoA oxidase is considered as residual oxygen consumption rather than cell respiration. |
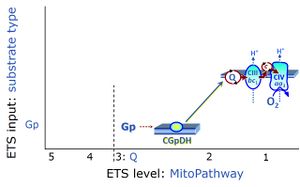
| Glycerophosphate pathway control state | Gp | The glycerophosphate pathway control state (Gp) is an ET-pathway level 3 control state, supported by the fuel substrate glycerophosphate and electron transfer through glycerophosphate dehydrogenase Complex into the Q-junction. The glycerolphosphate shuttle represents an important pathway, particularly in liver and blood cells, of making cytoplasmic NADH available for mitochondrial oxidative phosphorylation. Cytoplasmic NADH reacts with dihydroxyacetone phosphate catalyzed by cytoplasmic glycerophos-phate dehydrogenase. On the outer face of the inner mitochondrial membrane, mitochondrial glycerophosphate dehydrogenase oxidises glycerophosphate back to dihydroxyacetone phosphate, a reaction not generating NADH but reducing a flavin prosthesic group. The reduced flavoprotein donates its reducing equivalents to the electron transfer-pathway at the level of CoQ. |
| LEAK respiration | L | |
| LEAK state with ATP | L(T) | |
| LEAK state with oligomycin | L(Omy) | |
| LEAK state without adenylates | L(n) | |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
|
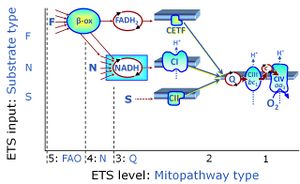
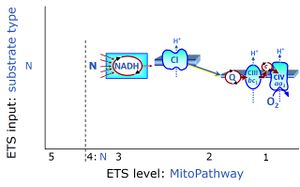
| NADH electron transfer-pathway state | N | The NADH electron transfer-pathway state (N) is obtained by addition of NADH-linked substrates (CI-linked), feeding electrons into the N-junction catalyzed by various mt-dehydrogenases. N-supported flux is induced in mt-preparations by the addition of NADH-generating substrate combinations of pyruvate (P), glutamate (G), malate (M), oxaloacetate (Oa), oxoglutarate (Og), citrate, hydroxybutyrate. These N-junction substrates are (indirectly) linked to Complex I by the corresponding dehydrogenase-catalyzed reactions reducing NAD+ to NADH+H+ + H+. The most commonly applied N-junction substrate combinations are: PM, GM, PGM. The malate-anaplerotic pathway control state (M alone) is a special case related to malic enzyme (mtME). The glutamate-anaplerotic pathway control state (G alone) supports respiration through glutamate dehydrogenase (mtGDH). Oxidation of tetrahydrofolate is a NAD(P)H linked pathway with formation of formate. In mt-preparations, succinate dehydrogenase (SDH; CII) is largely substrate-limited in N-linked respiration, due to metabolite depletion into the incubation medium. The residual involvement of S-linked respiration in the N-pathway control state can be further suppressed by the CII-inhibitor malonic acid). In the N-pathway control state ET pathway level 4 is active. |
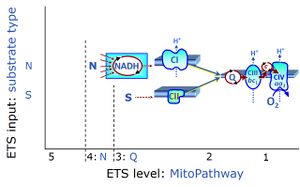
| NS-pathway control state | NS, CI&II | NS-pathway control is exerted in the NS-linked substrate state (flux in the NS-linked substrate state, NS; or Complex I&II, CI&II-linked substrate state). NS-OXPHOS capacity provides an estimate of physiologically relevant maximum mitochondrial respiratory capacity. NS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state in combination with succinate (Succinate pathway; S). Whereas NS expresses substrate control in terms of substrate types (N and S), CI&II defines the same concept in terms of convergent electron transfer to the Q-junction (pathway control). NS is the abbreviation for the combination of NADH-linked substrates (N) and succinate (S). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. NS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| Noncoupled respiration | E | Noncoupled respiration is maximum electron flow in an open-transmembrane proton circuit mode of operation (see ET capacity). » MiPNet article |
| OXPHOS capacity | P | |
| Oxidative phosphorylation | OXPHOS | |
| R-L net ROUTINE capacity | R-L | |
| ROUTINE respiration | R | |
| Reference state | Z | The reference state Z (reference rate ZX) is the respiratory state with high flux in relation to the background state Y with low background flux YX. The transition between the background state and the reference state is a step brought about by a metabolic control variable X. If X stimulates flux (ADP, fuel substrate), it is present in the reference state but absent in the background state. If X is an inhibitor of flux, it is absent in the reference state but present in the background state. The reference state is specific for a single step to define the flux control efficiency. In contrast, in a sequence of multiple steps, the common reference state is frequently taken as the state with the highest flux in the entire sequence, as used in the definition of the flux control ratio. |
| Residual endogenous substrates | REN, Ren |  Oxygen consumption due to residual endogenous substrates. Ren is the respiration in the REN state. It is due to oxidative reactions in mt-preparations incubated without addition of fuel substrates in the absence or presence of ADP (in the presence of ADP to stimulate the consumption of endogenous fuel substrates: State 2). Ren values may be used as technical replicates when obtained from the same mt-preparation in different protocols. Oxygen consumption due to residual endogenous substrates. Ren is the respiration in the REN state. It is due to oxidative reactions in mt-preparations incubated without addition of fuel substrates in the absence or presence of ADP (in the presence of ADP to stimulate the consumption of endogenous fuel substrates: State 2). Ren values may be used as technical replicates when obtained from the same mt-preparation in different protocols.
Ren may be higher than Rox. Correspondingly, Q and NAD are not fully oxidized in the REN state compared to the ROX state. In previous editions (including Gnaiger 2020 BEC MitoPathways), the REN state was not distinguished from the ROX state. However, in novel applications (Q-Module and NADH-Module), a distinction of these states is necessary. Care must be taken when assuming Ren as a substitute of Rox correction of mitochondrial respiration. |
| Residual oxygen consumption | ROX, Rox |  Residual oxygen consumption Rox — respiration in the ROX state — is due to oxidative side reactions remaining after inhibition of the electron transfer pathway (ET pathway) in mitochondrial preparations or living cells. Different conditions designated as ROX states (different combinations of inhibitors of CI, CII, CIII and CIV) may result in consistent or significantly different levels of oxygen consumption. Hence the best quantitative estimate of Rox has to be carefully evaluated. Mitochondrial respiration is frequently corrected for Rox as the baseline state. Then, total ROUTINE, LEAK respiration, OXPHOS or ET (R, L, P and E) respiration are distinguished from the corresponding Rox-corrected, mitochondrial (ET-pathway linked) fluxes: R(mt), L(mt), P(mt) and E(mt). Alternatively, R, L, P and E are defined as Rox-corrected rates, in contrast to total rates R´, L´, P´ and E´. When expressing Rox as a fraction of ET capacity (flux control ratio), total flux E´ (not corrected for Rox), should be taken as the reference. Rox may be related to, but is of course different from ROS production.
In previous editions, (including Gnaiger 2020 BEC MitoPathways), the REN state was not distinguished from the ROX state. However, in novel applications (Q-Module and NADH-Module), a distinction of these states is necessary. Care must be taken when assuming Ren as a substitute of Rox correction of mitochondrial respiration. Residual oxygen consumption Rox — respiration in the ROX state — is due to oxidative side reactions remaining after inhibition of the electron transfer pathway (ET pathway) in mitochondrial preparations or living cells. Different conditions designated as ROX states (different combinations of inhibitors of CI, CII, CIII and CIV) may result in consistent or significantly different levels of oxygen consumption. Hence the best quantitative estimate of Rox has to be carefully evaluated. Mitochondrial respiration is frequently corrected for Rox as the baseline state. Then, total ROUTINE, LEAK respiration, OXPHOS or ET (R, L, P and E) respiration are distinguished from the corresponding Rox-corrected, mitochondrial (ET-pathway linked) fluxes: R(mt), L(mt), P(mt) and E(mt). Alternatively, R, L, P and E are defined as Rox-corrected rates, in contrast to total rates R´, L´, P´ and E´. When expressing Rox as a fraction of ET capacity (flux control ratio), total flux E´ (not corrected for Rox), should be taken as the reference. Rox may be related to, but is of course different from ROS production.
In previous editions, (including Gnaiger 2020 BEC MitoPathways), the REN state was not distinguished from the ROX state. However, in novel applications (Q-Module and NADH-Module), a distinction of these states is necessary. Care must be taken when assuming Ren as a substitute of Rox correction of mitochondrial respiration. |
| Respiratory state | Respiratory states of mitochondrial preparations and living cells are defined in the current literature in many ways and with a diversity of terms. Mitochondrial respiratory states must be defined in terms of both, the coupling-control state and the electron-transfer-pathway state. | |
| Resting metabolic rate | RMR | Resting respiration or resting metabolic rate (RMR) is measured under standard conditions of an 8–12-h fast and a 12-h abstinence from exercise. In an exemplary study (Haugen 2003 Am J Clin Nutr), "subjects rested quietly in the supine position in an isolated room with the temperature controlled to 21–24° C. RMR was measured for 15–20 min. Criteria for a valid RMR was a minimum of 15 min of steady state, determined as a <10% fluctuation in oxygen consumption and <5% fluctuation in respiratory quotient". The main difference between RMR and BMR (basal metabolic rate) is the position of the subject during measurement. Resting metabolic rate is the largest component of the daily energy budget in most human societies and increases with physical training state (Speakman 2003 Proc Nutr Soc). |
| SGp-pathway control state | SGp | SGp: Succinate & Glycerophosphate.
MitoPathway control state: SGp; obtained with OctPGMSGp(Rot) SUIT protocol: SUIT-001 and ((SUIT-002 |
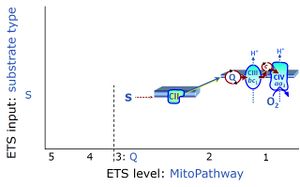
| Succinate pathway | S, SRot |
The Succinate pathway (S-pathway; S) is the electron transfer pathway that supports succinate-linked respiration (succinate-induced respiratory state; previously used nomenclature: CII-linked respiration; SRot; see Gnaiger 2009 Int J Biochem Cell Biol). The S-pathway describes the electron flux through Complex II (CII; see succinate dehydrogenase, SDH) from succinate and FAD to fumarate and CII-bound flavin adenine dinucleotide (FADH2) to the Q-junction. The S-pathway control state is usually induced in mt-preparations by addition of succinate&rotenone. In this case, only Complex III and Complex IV are involved in pumping protons from the matrix (positive phase, P-phase) to the negative phase (N-phase) with a P»/O2 of 3.5 (P»/O ratio = 1.75). |
Respiratory states: find synonyms, historically used and ambiguous terms
| Term | Abbreviation | Redirected to |
|---|---|---|
| Complex I&II-linked substrate state | NS | See NS-pathway control state (previous: CI&II-linked) |
| Complex I-linked substrate state | N | See N-pathway control state (previous: CI-linked) versus Complex I |
| Complex II-linked substrate state | SRot, S | See S-pathway control state (previous: CII-linked) |
| ET-pathway competent state | Electron transfer pathway competent state, see Electron-transfer-pathway state. | |
| Level flow | E | |
| State 1 | State 1 is the first respiratory state in an oxygraphic protocol described by Chance and Williams (1955), when isolated mitochondria are added to mitochondrial respiration medium containing oxygen and inorganic phosphate, but no ADP and no reduced respiratory substrates. In State 1, LEAK respiration may be supported to some extent by undefined endogenous substrates, which are oxidized and slowly exhausted. After oxidation of endogenous substrates, only residual oxygen consumption remains (ROX). | |
| State 2 | ROXD |  Substrate limited state of residual oxygen consumption, after addition of ADP to isolated mitochondria suspended in mitochondrial respiration medium in the absence of reduced substrates (ROXD). Residual endogenous substrates are oxidized during a transient stimulation of oxygen flux by ADP. The peak – supported by endogenous substrates – is, therefore, a pre-steady state phenomenon preceding State 2. Subsequently oxygen flux declines to a low level (or zero) at the steady State 2 (Chance and Williams 1955). ADP concentration (D) remains high during ROXD. Substrate limited state of residual oxygen consumption, after addition of ADP to isolated mitochondria suspended in mitochondrial respiration medium in the absence of reduced substrates (ROXD). Residual endogenous substrates are oxidized during a transient stimulation of oxygen flux by ADP. The peak – supported by endogenous substrates – is, therefore, a pre-steady state phenomenon preceding State 2. Subsequently oxygen flux declines to a low level (or zero) at the steady State 2 (Chance and Williams 1955). ADP concentration (D) remains high during ROXD. |
| State 3 | P | |
| State 3u | E | |
| State 4 | LT | |
| State 5 | State 5 is the respiratory state obtained in a protocol with isolated mitochondria after a sequence of State 1 to State 4, when the concentration of O2 is depleted in the closed oxygraph chamber and zero oxygen (the anaerobic state) is reached (Chance and Williams, 1955; Table I). State 5 is defined in the original publication in two ways: State 5 may be obtained by antimycin A treatment or by anaerobiosis (Chance and Williams, 1955; page 414). Antimycin A treatment yields a State 5 equivalent to a state for measurement of residual oxygen consumption, ROX (which may also be induced by rotenone+myxothiazol; Gnaiger 2009). Setting State 5 equivalent to ROX or anoxia (Chance and Williams 1955) can be rationalized only in the context of measurement of cytochrome redox states, whereas in the context of respiration State 5 is usually referred to as anoxic. | |
| Static head | L | |
| Substrate control state | See Electron-transfer-pathway state |
References
| Bioblast link | Reference | Year |
|---|---|---|
| Chance 1955 J Biol Chem-III | Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation: III. The steady state. J Biol Chem 217:409-27. | 1955 |
| Chance 1955 J Biol Chem-I | Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383-93. | 1955 |
| Chance 1956 Adv Enzymol Relat Subj Biochem | Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65-134. | 1956 |
| Gnaiger 2009 Int J Biochem Cell Biol | Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. https://doi.org/10.1016/j.biocel.2009.03.013 | 2009 |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |